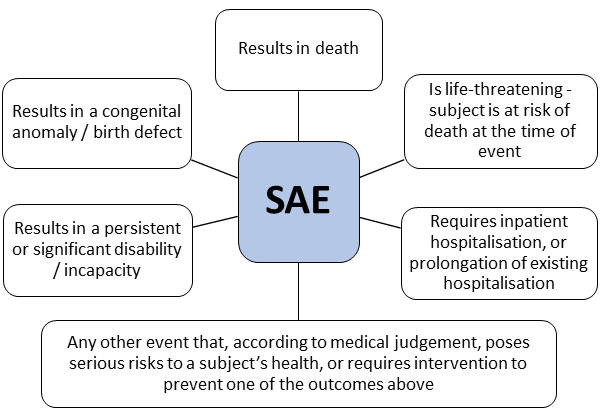
An ‘adverse event’ (AE) is any untoward (i.e. unfavourable and unintended) medical occurrence in a research subject.
It may or may not be caused by any research procedures or interventions, but is nonetheless given importance because its timing of occurrence corresponded with the study.