The research protocol is usually accompanied by some or all of the following documents, depending on the type of research you are doing. These may be incorporated within the research protocol or kept as separate documents.
Like the research protocol, you can still make changes to these documents once you embark on your study, subject to further approval by the research ethics committee.
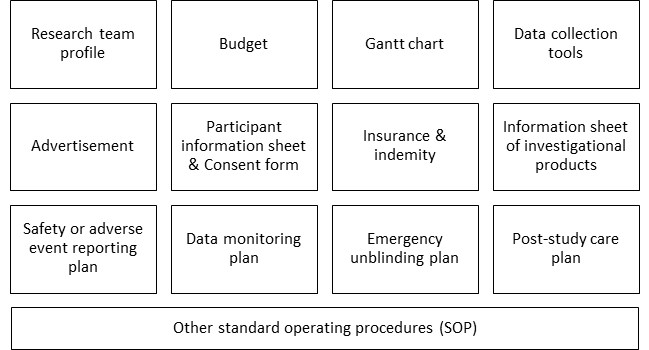
Put together, the research protocol and all protocol-related documents should demonstrate to a reviewer that your study is well-planned, feasible, scientifically sound, beneficial and safe.
We will go through each of the above and explain their significance from the perspectives of an ethics committee.